By William L. Diehl, M.D.
From time to time I will present topics that are not specific to Triple Negative Breast Cancer (TNBC) but of importance to all types of breast cancer. Today I will talk about Liquid Biopsies. These types of biopsies detect cancer through a blood test.
When cancers grow, they shed cells into the blood-stream (Circulating Tumor Cells or CTC’s). Also, when a cancer cell dies, it releases bits and pieces of its DNA into the blood providing what is called Circulating Tumor DNA or ctDNA. Both of these can be captured and analyzed in the lab.
Advantages in Triple Negative Breast Cancer
Imagine a situation where you went to the lab and gave a sample of blood to diagnose an early cancer. Detection of triple negative breast cancer is one of the potential advantages of liquid biopsies but there are others. They can also be used to monitor whether surgery or chemotherapy are effective. You could compare the characteristics of the liquid biopsy to those of the original tumor. This might show a change in the cancer. Doctors can study how treatment affects the biology of the cancer. One could also analyze the characteristics of the CTC’s or ctDNA and identify proteins that could be targeted by immunotherapy. After treatment, liquid biopsies could be used to monitor patients to see if the cancer returns.
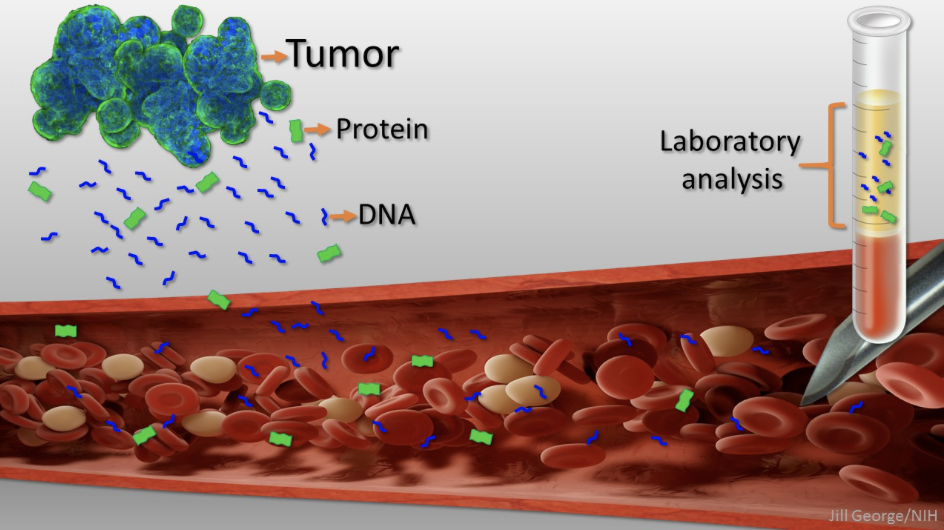
In a multi-center clinical trial, researchers from Johns Hopkins Cancer Center recently screened approximately 9,991 healthy women using their liquid biopsy test named CancerSEEK. They also used conventional screening tests. CancerSEEK identified 26 new cancer patients and conventional screening an additional 24. This suggests liquid biopsies may be an important addition to conventional screening methods. Of note, 65% of the cancers identified by the blood test were early stage cancers. This trial was not limited to triple negative breast cancers, but the liquid biopsy was concordant with the primary cancer every time.
Pitfalls of Liquid Biopsies
These tests are not quite ready for prime time. False positive results may lead to anxiety and the need for additional radiographic or laboratory studies. They may even lead to invasive procedures in an effort to find a cancer that is not there. We are in the early stages of gathering clinical information regarding this exciting new technology. I suspect that in the future the combination of liquid biopsies and conventional screening studies will become the standard of care. They will also become part of the process we use to monitor the effectiveness of treatment and to detect recurrences. So for now, we will continue to monitor the progress of these types of biopsies and hope they live up to their exciting potential.